COVID-19 “CORONAVIRUS”
IGG/IGM RAPID TEST KIT
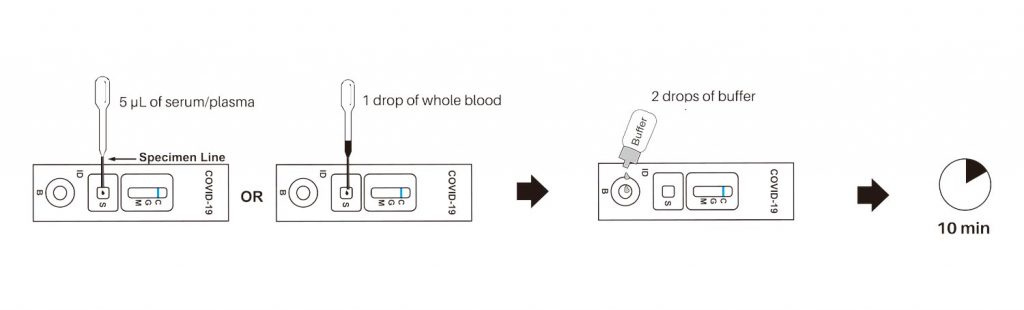
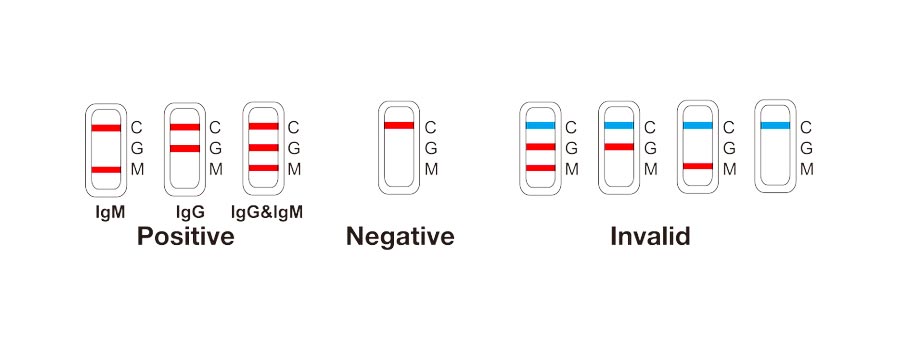
The COVID-19 IgG/IgM (Whole Blood/Serum/Plasma) Rapid Test Device utilizes lateral flow technology for the qualitative, differential detection of both anti-SARS-CoV-2 IgM and IgG antibodies. In general, antibodies can be detected 1-3 weeks after infection. This test is intended to screen patients for SARS-CoV-2 antibodies.
Our easy-to-use COVID-19 test kit provides results in just 10 minutes
Cost-Effective
Fast
Accurate
Features
1 These tests are authorized by the FDA for emergency use as of May 29, 2020. Forensic/medical professional only: Tests should be conducted by a licensed phlebotomist or a medical professional.
2 Forensic/medical professional use only: Tests should be conducted by a licensed phlebotomist or a medical professional. Verification of use case prior to shipping is mandatory. Verification of use case prior to shipping is mandatory.
3 Please defer to your local regulations.
HOW ACCURATE IS THE CORONAVIRUS
(COVID-19) RAPID TEST?
Our test has shown in clinical evaluation to have a total agreement of 97.19% and a kappa value of 0.94.